Los valores de datos de los potenciales de electrodo estándar (E°) se dan en la siguiente tabla, en voltios en relación con el electrodo de hidrógeno estándar, y son para las siguientes condiciones:
- Una temperatura de 298.15 K (25.00 °C; 77.00 °F).
- Una concentración efectiva de 1 ml/L para cada especie acuosa o una especie en un amalgama de mercurio (una aleación de mercurio con otro metal).
- Presión parcial de 101.325 kPa (absoluto) (1 atm, 1.01325 bar) para cada reactivo gaseoso. Esta presión se utiliza porque la mayoría de los datos de literatura todavía se dan para este valor (1 am) en lugar de para el estándar actual de 100 kPa (1 bar) actualmente considerado en el estado estándar.
- Actividad de unidad para cada líquido puro sólido, puro o para el agua (solvente). La relación en potencial electrodo de metales en agua salada (como electrolito) se da en el serie galvanic.
- Aunque muchas de las medias células están escritas para transferencias multielectrónicas, los potenciales tabulados son para una transferencia de un solo electrón. Todas las reacciones deben dividirse por el coeficiente estoquiométrico para que el electron obtenga la correspondiente ecuación de reacción corregida. Por ejemplo, la ecuación Fe2+ + 2e− Fe Fes) (–0.44 V) significa que requiere 2 × 0.44 eV = 0.88 eV de energía para ser absorbido (de ahí el signo menos) con el fin de crear un átomo neutral de Fe(sDe una Fe2+ iones y dos electrones, o 0,44 eV por electron, que es 0,44 J/C de electrones, que es 0,44 V.
- Después de dividir por el número de electrones, el potencial estándar E° está relacionado con el estándar Gibbs energía libre de formación ΔGf° por:
E=.. Δ Δ Gizquierda− − .. Δ Δ GderechoF{displaystyle E={fracsum Delta G_{text{left}-sum ¿Qué?

- La ecuación de Nernst dará potenciales a concentraciones, presiones y temperaturas distintas de las estándar.
- Tenga en cuenta que el cuadro puede carecer de coherencia debido a datos de diferentes fuentes. Por ejemplo:
Cu+
| + e− | ⇌ | Cu(s) | ()E
1 = +0,520 V)
|
| Cu2+ | + 2e− | ⇌ | Cu(s) | ()E
2 = +0.337 V)
|
| Cu2+ | + e− | ⇌ | Cu+
| ()E
3 = +0.159 V)
|
- Calculando el potencial usando la energía libre de Gibbs (E
3 = 2E
2 – E
1) da el potencial para E
3 como 0.154 V, no el valor experimental de 0.159 V.
Tabla de potenciales de electrodo estándar
Leyenda: (s) – sólido; (l) – líquido; (g) – gas; (aq) – acuoso (predeterminado para todas las especies cargadas); (Hg) – amalgama; negrita: ecuaciones de electrólisis del agua.
| Element
|
Half-reaction
|
E° / V
|
Electrons
|
Ref.
|
| Oxidant
|
⇌
|
Reductant
|
| &
|
-9
|
| Zz
|
9
|
| Sr
|
Sr+
+ e−
|
⇌
|
Sr(s)
|
-4.101
|
1
|
|
| Ca
|
Ca+
+ e−
|
⇌
|
Ca(s)
|
-3.8
|
1
|
|
| Th
|
Th4+ + e−
|
⇌
|
Th3+
|
-3.6
|
1
|
|
| Pr
|
Pr3+ + e−
|
⇌
|
Pr2+
|
-3.1
|
1
|
|
| N
|
3N2(g) + 2 H+ + 2 e−
|
⇌
|
2HN3(aq)
|
-3.09
|
2
|
|
| Li
|
Li+ + e−
|
⇌
|
Li(s)
|
-3.0401
|
1
|
|
| N
|
N2(g) + 4H2O + 2 e−
|
⇌
|
2NH2OH(aq) + 2 OH−
|
-3.04
|
2
|
|
| Cs
|
Cs+ + e−
|
⇌
|
Cs(s)
|
-3.026
|
1
|
|
| Ca
|
Ca(OH)2 + 2 e−
|
⇌
|
Ca(s) + 2 OH−
|
-3.02
|
2
|
|
| Er
|
Er3+ + e−
|
⇌
|
Er2+
|
-3
|
1
|
|
| Ba
|
Ba(OH)2 + 2 e−
|
⇌
|
Ba(s) + 2 OH−
|
-2.99
|
2
|
|
| Rb
|
Rb+ + e−
|
⇌
|
Rb(s)
|
-2.98
|
1
|
|
| K
|
K+ + e−
|
⇌
|
K(s)
|
-2.931
|
1
|
|
| Ba
|
Ba2+ + 2 e−
|
⇌
|
Ba(s)
|
-2.912
|
2
|
|
| La
|
La(OH)3(s) + 3 e−
|
⇌
|
La(s) + 3 OH−
|
-2.9
|
3
|
|
| Fr
|
Fr+ + e−
|
⇌
|
Fr(s)
|
-2.9
|
1
|
|
| Sr
|
Sr2+ + 2 e−
|
⇌
|
Sr(s)
|
-2.899
|
2
|
|
| Sr
|
Sr(OH)2 + 2 e−
|
⇌
|
Sr(s) + 2 OH−
|
-2.88
|
2
|
|
| Ca
|
Ca2+ + 2 e−
|
⇌
|
Ca(s)
|
-2.868
|
2
|
|
| Li
|
Li+
+ C
6(s) + e−
|
⇌
|
LiC6(s)
|
-2.84
|
1
|
|
| Eu
|
Eu2+ + 2 e−
|
⇌
|
Eu(s)
|
-2.812
|
2
|
|
| Ra
|
Ra2+ + 2 e−
|
⇌
|
Ra(s)
|
-2.8
|
2
|
|
| Ho
|
Ho3+ + e−
|
⇌
|
Ho2+
|
-2.8
|
1
|
|
| Bk
|
Bk3+ + e−
|
⇌
|
Bk2+
|
-2.8
|
1
|
|
| Yb
|
Yb2+ + 2 e−
|
⇌
|
Yb(s)
|
-2.76
|
2
|
|
| Na
|
Na+ + e−
|
⇌
|
Na(s)
|
-2.71
|
1
|
|
| Mg
|
Mg+ + e−
|
⇌
|
Mg(s)
|
-2.7
|
1
|
|
| Nd
|
Nd3+ + e−
|
⇌
|
Nd2+
|
-2.7
|
1
|
|
| Mg
|
Mg(OH)
2 + 2 e−
|
⇌
|
Mg(s) + 2 OH−
|
-2.69
|
2
|
|
| Sm
|
Sm2+ + 2 e−
|
⇌
|
Sm(s)
|
-2.68
|
2
|
|
| Be
|
Be
2O2−
3 + 3H2O + 4 e−
|
⇌
|
2Be(s) + 6 OH−
|
-2.63
|
4
|
|
| Pm
|
Pm3+ + e−
|
⇌
|
Pm2+
|
-2.6
|
1
|
|
| Dy
|
Dy3+ + e−
|
⇌
|
Dy2+
|
-2.6
|
1
|
|
| No
|
No2+ + 2 e−
|
⇌
|
No
|
-2.5
|
2
|
|
| Hf
|
HfO(OH)
2 + H2O + 4 e−
|
⇌
|
Hf(s) + 4 OH−
|
-2.5
|
4
|
|
| Th
|
Th(OH)
4 + 4 e−
|
⇌
|
Th(s) + 4 OH−
|
-2.48
|
4
|
|
| Md
|
Md2+
+ 2 e−
|
⇌
|
Md
|
-2.4
|
2
|
|
| Tm
|
Tm2+
+ 2 e−
|
⇌
|
Tm(s)
|
-2.4
|
2
|
|
| La
|
La3+ + 3 e−
|
⇌
|
La(s)
|
-2.379
|
3
|
|
| Y
|
Y3+ + 3 e−
|
⇌
|
Y(s)
|
-2.372
|
3
|
|
| Mg
|
Mg2+
+ 2 e−
|
⇌
|
Mg(s)
|
-2.372
|
2
|
|
| Zr
|
ZrO(OH)2(s) + H2O + 4 e−
|
⇌
|
Zr(s) + 4 OH−
|
-2.36
|
4
|
|
| Pr
|
Pr3+ + 3 e−
|
⇌
|
Pr(s)
|
-2.353
|
3
|
|
| Ce
|
Ce3+ + 3 e−
|
⇌
|
Ce(s)
|
-2.336
|
3
|
|
| Er
|
Er3+ + 3 e−
|
⇌
|
Er(s)
|
-2.331
|
3
|
|
| Ho
|
Ho3+ + 3 e−
|
⇌
|
Ho(s)
|
-2.33
|
3
|
|
| Al
|
H
2AlO−
3 + H2O + 3 e−
|
⇌
|
Al(s) + 4 OH−
|
-2.33
|
3
|
|
| Nd
|
Nd3+ + 3 e−
|
⇌
|
Nd(s)
|
-2.323
|
3
|
|
| Tm
|
Tm3+ + 3 e−
|
⇌
|
Tm(s)
|
-2.319
|
3
|
|
| Al
|
Al(OH)
3(s) + 3 e−
|
⇌
|
Al(s) + 3 OH−
|
-2.31
|
3
|
|
| Sm
|
Sm3+ + 3 e−
|
⇌
|
Sm(s)
|
-2.304
|
3
|
|
| Fm
|
Fm2+ + 2 e−
|
⇌
|
Fm
|
-2.3
|
2
|
|
| Am
|
Am3+ + e−
|
⇌
|
Am2+
|
-2.3
|
1
|
|
| Dy
|
Dy3+ + 3 e−
|
⇌
|
Dy(s)
|
-2.295
|
3
|
|
| Lu
|
Lu3+ + 3 e−
|
⇌
|
Lu(s)
|
-2.28
|
3
|
|
| Tb
|
Tb3+ + 3 e−
|
⇌
|
Tb(s)
|
-2.28
|
3
|
|
| Gd
|
Gd3+ + 3 e−
|
⇌
|
Gd(s)
|
-2.279
|
3
|
|
| H
|
H2(g) + 2 e−
|
⇌
|
2H−
|
-2.23
|
2
|
|
| Es
|
Es2+
+ 2 e−
|
⇌
|
Es(s)
|
-2.23
|
2
|
|
| Pm
|
Pm2+
+ 2 e−
|
⇌
|
Pm(s)
|
-2.2
|
2
|
|
| Tm
|
Tm3+ + e−
|
⇌
|
Tm2+
|
-2.2
|
1
|
|
| Dy
|
Dy2+
+ 2 e−
|
⇌
|
Dy(s)
|
-2.2
|
2
|
|
| Ac
|
Ac3+ + 3 e−
|
⇌
|
Ac(s)
|
-2.2
|
3
|
|
| Yb
|
Yb3+ + 3 e−
|
⇌
|
Yb(s)
|
-2.19
|
3
|
|
| Cf
|
Cf2+
+ 2 e−
|
⇌
|
Cf(s)
|
-2.12
|
2
|
|
| Nd
|
Nd2+
+ 2 e−
|
⇌
|
Nd(s)
|
-2.1
|
2
|
|
| Ho
|
Ho2+
+ 2 e−
|
⇌
|
Ho(s)
|
-2.1
|
2
|
|
| Sc
|
Sc3+ + 3 e−
|
⇌
|
Sc(s)
|
-2.077
|
3
|
|
| Al
|
AlF3−6 + 3 e−
|
⇌
|
Al(s) + 6F−
|
-2.069
|
3
|
|
| Am
|
Am3+ + 3 e−
|
⇌
|
Am(s)
|
-2.048
|
3
|
|
| Cm
|
Cm3+ + 3 e−
|
⇌
|
Cm(s)
|
-2.04
|
3
|
|
| Pu
|
Pu3+ + 3 e−
|
⇌
|
Pu(s)
|
-2.031
|
3
|
|
| Pr
|
Pr2+
+ 2 e−
|
⇌
|
Pr(s)
|
-2
|
2
|
|
| Er
|
Er2+
+ 2 e−
|
⇌
|
Er(s)
|
-2
|
2
|
|
| Eu
|
Eu3+ + 3 e−
|
⇌
|
Eu(s)
|
-1.991
|
3
|
|
| Lr
|
Lr3+ + 3 e−
|
⇌
|
Lr
|
-1.96
|
3
|
|
| Cf
|
Cf3+ + 3 e−
|
⇌
|
Cf(s)
|
-1.94
|
3
|
|
| Es
|
Es3+ + 3 e−
|
⇌
|
Es(s)
|
-1.91
|
3
|
|
| Pa
|
Pa4+
+ e−
|
⇌
|
Pa3+
|
-1.9
|
1
|
|
| Am
|
Am2+
+ 2 e−
|
⇌
|
Am(s)
|
-1.9
|
2
|
|
| Th
|
Th4+
+ 4 e−
|
⇌
|
Th(s)
|
-1.899
|
4
|
|
| Fm
|
Fm3+ + 3 e−
|
⇌
|
Fm
|
-1.89
|
3
|
|
| Np
|
Np3+ + 3 e−
|
⇌
|
Np(s)
|
-1.856
|
3
|
|
| Be
|
Be2+
+ 2 e−
|
⇌
|
Be(s)
|
-1.847
|
2
|
|
| P
|
H
2PO−
2 + e−
|
⇌
|
P(s) + 2 OH−
|
-1.82
|
1
|
|
| U
|
U3+ + 3 e−
|
⇌
|
U(s)
|
-1.798
|
3
|
|
| Sr
|
Sr2+
+ 2 e−
|
⇌
|
Sr(Hg)
|
-1.793
|
2
|
|
| B
|
H
2BO−
3 + H2O + 3 e−
|
⇌
|
B(s) + 4 OH−
|
-1.79
|
3
|
|
| Th
|
ThO2 + 4 H+ + 4 e−
|
⇌
|
Th(s) + 2H2O
|
-1.789
|
4
|
|
| Hf
|
HfO2+
+ 2 H+ + 4 e−
|
⇌
|
Hf(s) + H2O
|
-1.724
|
4
|
|
| P
|
HPO2−
3 + 2H2O + 3 e−
|
⇌
|
P(s) + 5 OH−
|
-1.71
|
3
|
|
| Si
|
SiO2−3 + 3H2O + 4 e−
|
⇌
|
Si(s) + 6 OH−
|
-1.697
|
4
|
|
| Al
|
Al3+ + 3 e−
|
⇌
|
Al(s)
|
-1.662
|
3
|
|
| Ti
|
Ti2+
+ 2 e−
|
⇌
|
Ti(s)
|
-1.63
|
2
|
|
| Zr
|
ZrO
2(s) + 4 H+ + 4 e−
|
⇌
|
Zr(s) + 2H2O
|
-1.553
|
4
|
|
| Zr
|
Zr4+
+ 4 e−
|
⇌
|
Zr(s)
|
-1.45
|
4
|
|
| Ti
|
Ti3+
+ 3 e−
|
⇌
|
Ti(s)
|
-1.37
|
3
|
|
| Ti
|
TiO(s) + 2 H+ + 2 e−
|
⇌
|
Ti(s) + H2O
|
-1.31
|
2
|
|
| Ti
|
Ti2O3(s) + 2 H+ + 2 e−
|
⇌
|
2TiO(s) + H2O
|
-1.23
|
2
|
|
| Zn
|
Zn(OH)2−
4 + 2 e−
|
⇌
|
Zn(s) + 4 OH−
|
-1.199
|
2
|
|
| Mn
|
Mn2+
+ 2 e−
|
⇌
|
Mn(s)
|
-1.185
|
2
|
|
| Fe
|
Fe(CN)4−
6 + 6 H+ + 2 e−
|
⇌
|
Fe(s) + 6HCN(aq)
|
-1.16
|
2
|
|
| Te
|
Te(s) + 2 e−
|
⇌
|
Te2−
|
-1.143
|
2
|
|
| V
|
V2+
+ 2 e−
|
⇌
|
V(s)
|
-1.13
|
2
|
|
| Nb
|
Nb3+ + 3 e−
|
⇌
|
Nb(s)
|
-1.099
|
3
|
|
| Sn
|
Sn(s) + 4 H+ + 4 e−
|
⇌
|
SnH
4(g)
|
-1.07
|
4
|
|
| Ti
|
TiO2+
+ 2 H+ + 4 e−
|
⇌
|
Ti(s) + H2O
|
-0.93
|
4
|
|
| Si
|
SiO
2(s) + 4 H+ + 4 e−
|
⇌
|
Si(s) + 2H2O
|
-0.91
|
4
|
|
| B
|
B(OH)3(aq) + 3 H+ + 3 e−
|
⇌
|
B(s) + 3H2O
|
-0.89
|
3
|
|
| Fe
|
Fe(OH)
2(s) + 2 e−
|
⇌
|
Fe(s) + 2 OH−
|
-0.89
|
2
|
|
| Fe
|
Fe
2O
3(s) + 3H2O + 2 e−
|
⇌
|
2Fe(OH)
2(s) + 2 OH−
|
-0.86
|
2
|
|
| H
|
2H2O + 2 e−
|
⇌
|
H
2(g) + 2 OH−
|
-0.8277
|
2
|
|
| Bi
|
Bi(s) + 3 H+ + 3 e−
|
⇌
|
BiH3
|
-0.8
|
3
|
|
| Zn
|
Zn2+
+ 2 e−
|
⇌
|
Zn(Hg)
|
-0.7628
|
2
|
|
| Zn
|
Zn2+
+ 2 e−
|
⇌
|
Zn(s)
|
-0.7618
|
2
|
|
| Ta
|
Ta2O5(s) + 10 H+ + 10 e−
|
⇌
|
2Ta(s) + 5H2O
|
-0.75
|
10
|
|
| Cr
|
Cr3+ + 3 e−
|
⇌
|
Cr(s)
|
-0.74
|
3
|
|
| Ni
|
Ni(OH)2(s) + 2 e−
|
⇌
|
Ni(s) + 2 OH−
|
-0.72
|
2
|
|
| Ag
|
Ag2S(s) + 2 e−
|
⇌
|
2Ag(s) + S2−
(aq)
|
-0.69
|
2
|
|
| Au
|
[Au(CN)
2]−
+ e−
|
⇌
|
Au(s) + 2CN−
|
-0.6
|
1
|
|
| Ta
|
Ta3+
+ 3 e−
|
⇌
|
Ta(s)
|
-0.6
|
3
|
|
| Pb
|
PbO(s) + H2O + 2 e−
|
⇌
|
Pb(s) + 2 OH−
|
-0.58
|
2
|
|
| Ti
|
2TiO
2(s) + 2 H+ + 2 e−
|
⇌
|
Ti
2O
3(s) + H2O
|
-0.56
|
2
|
|
| Ga
|
Ga3+ + 3 e−
|
⇌
|
Ga(s)
|
-0.53
|
3
|
|
| U
|
U4+
+ e−
|
⇌
|
U3+
|
-0.52
|
1
|
|
| P
|
H3PO2(aq) + H+ + e−
|
⇌
|
P(white) + 2H2O
|
-0.508
|
1
|
|
| P
|
H
3PO
3(aq) + 2 H+ + 2 e−
|
⇌
|
H
3PO
2(aq) + H2O
|
-0.499
|
2
|
|
| Ni
|
NiO
2(s) + 2H2O + 2 e−
|
⇌
|
Ni(OH)2(s) + 2 OH−
|
-0.49
|
2
|
|
| P
|
H3PO3(aq) + 3 H+ + 3 e−
|
⇌
|
P(red) + 3H2O
|
-0.454
|
3
|
|
| Cu
|
Cu(CN)−
2 + e−
|
⇌
|
Cu(s) + 2CN−
|
-0.44
|
1
|
|
| Fe
|
Fe2+
+ 2 e−
|
⇌
|
Fe(s)
|
-0.44
|
2
|
|
| C
|
2CO2(g) + 2 H+ + 2 e−
|
⇌
|
HOOCCOOH(aq)
|
-0.43
|
2
|
|
| Cr
|
Cr3+
+ e−
|
⇌
|
Cr2+
|
-0.42
|
1
|
|
| Cd
|
Cd2+
+ 2 e−
|
⇌
|
Cd(s)
|
-0.4
|
2
|
|
| Ge
|
GeO2(s) + 2 H+ + 2 e−
|
⇌
|
GeO(s) + H2O
|
-0.37
|
2
|
|
| Cu
|
Cu2O(s) + H2O + 2 e−
|
⇌
|
2Cu(s) + 2 OH−
|
-0.36
|
2
|
|
| Pb
|
PbSO4(s) + 2 e−
|
⇌
|
Pb(s) + SO2−
4
|
-0.3588
|
2
|
|
| Pb
|
PbSO
4(s) + 2 e−
|
⇌
|
Pb(Hg) + SO2−
4
|
-0.3505
|
2
|
|
| Eu
|
Eu3+
+ e−
|
⇌
|
Eu2+
|
-0.35
|
1
|
|
| In
|
In3+ + 3 e−
|
⇌
|
In(s)
|
-0.34
|
3
|
|
| Tl
|
Tl+
+ e−
|
⇌
|
Tl(s)
|
-0.34
|
1
|
|
| Ge
|
Ge(s) + 4 H+ + 4 e−
|
⇌
|
GeH
4(g)
|
-0.29
|
4
|
|
| Co
|
Co2+
+ 2 e−
|
⇌
|
Co(s)
|
-0.28
|
2
|
|
| P
|
H3PO4(aq) + 2 H+ + 2 e−
|
⇌
|
H
3PO
3(aq) + H2O
|
-0.276
|
2
|
|
| V
|
V3+ + e−
|
⇌
|
V2+
|
-0.26
|
1
|
|
| Ni
|
Ni2+ + 2 e−
|
⇌
|
Ni(s)
|
-0.25
|
2
|
|
| As
|
As(s) + 3 H+ + 3 e−
|
⇌
|
AsH3(g)
|
-0.23
|
3
|
|
| Ag
|
AgI(s) + e−
|
⇌
|
Ag(s) + I−
|
-0.15224
|
1
|
|
| Mo
|
MoO2(s) + 4 H+ + 4 e−
|
⇌
|
Mo(s) + 2H2O
|
-0.15
|
4
|
|
| Si
|
Si(s) + 4 H+ + 4 e−
|
⇌
|
SiH
4(g)
|
-0.14
|
4
|
|
| Sn
|
Sn2+
+ 2 e−
|
⇌
|
Sn(s)
|
-0.13
|
2
|
|
| O
|
O2(g) + H+ + e−
|
⇌
|
HO•
2(aq)
|
-0.13
|
1
|
|
| Pb
|
Pb2+ + 2 e−
|
⇌
|
Pb(s)
|
-0.126
|
2
|
|
| W
|
WO2(s) + 4 H+ + 4 e−
|
⇌
|
W(s) + 2H2O
|
-0.12
|
4
|
|
| P
|
P(red) + 3 H+ + 3 e−
|
⇌
|
PH3(g)
|
-0.111
|
3
|
|
| C
|
CO
2(g) + 2 H+ + 2 e−
|
⇌
|
HCOOH(aq)
|
-0.11
|
2
|
|
| Se
|
Se(s) + 2 H+ + 2 e−
|
⇌
|
H
2Se(g)
|
-0.11
|
2
|
|
| C
|
CO
2(g) + 2 H+ + 2 e−
|
⇌
|
CO(g) + H2O
|
-0.11
|
2
|
|
| Cu
|
Cu(NH
3)+
2 + e−
|
⇌
|
Cu(s) + 2NH
3(aq)
|
-0.1
|
1
|
|
| Sn
|
SnO(s) + 2 H+ + 2 e−
|
⇌
|
Sn(s) + H2O
|
-0.1
|
2
|
|
| Sn
|
SnO2(s) + 2 H+ + 2 e−
|
⇌
|
SnO(s) + H2O
|
-0.09
|
2
|
|
| W
|
WO3(aq) + 6 H+ + 6 e−
|
⇌
|
W(s) + 3H2O
|
-0.09
|
6
|
|
| Fe
|
Fe3O4(s) + 8 H+ + 8 e−
|
⇌
|
3Fe(s) + 4H2O
|
-0.085
|
8
|
|
| P
|
P(white) + 3 H+ + 3 e−
|
⇌
|
PH3(g)
|
-0.063
|
3
|
|
| Fe
|
Fe3+
+ 3 e−
|
⇌
|
Fe(s)
|
-0.04
|
3
|
|
| C
|
HCOOH(aq) + 2 H+ + 2 e−
|
⇌
|
HCHO(aq) + H2O
|
-0.03
|
2
|
|
| H
|
2 H+ + 2 e−
|
⇌
|
H
2(g)
|
0
|
2
|
|
| Ag
|
AgBr(s) + e−
|
⇌
|
Ag(s) + Br−
|
0.07133
|
1
|
|
| S
|
S4O2−6 + 2 e−
|
⇌
|
2S2O2−3
|
0.08
|
2
|
|
| N
|
N2(g) + 2H2O + 6 H+ + 6 e−
|
⇌
|
2NH4OH(aq)
|
0.092
|
6
|
|
| Hg
|
HgO(s) + H2O + 2 e−
|
⇌
|
Hg(l) + 2 OH−
|
0.0977
|
2
|
|
| Cu
|
Cu(NH
3)2+
4 + e−
|
⇌
|
Cu(NH
3)+
2 + 2NH
3(aq)
|
0.1
|
1
|
|
| Ru
|
Ru(NH3)3+6 + e−
|
⇌
|
Ru(NH
3)2+
6
|
0.1
|
1
|
|
| N
|
N2H4(aq) + 4H2O + 2 e−
|
⇌
|
2NH+
4 + 4 OH−
|
0.11
|
2
|
|
| Mo
|
H2MoO4(aq) + 6 H+ + 6 e−
|
⇌
|
Mo(s) + 4H2O
|
0.11
|
6
|
|
| Ge
|
Ge4+
+ 4 e−
|
⇌
|
Ge(s)
|
0.12
|
4
|
|
| C
|
C(s) + 4 H+ + 4 e−
|
⇌
|
CH4(g)
|
0.13
|
4
|
|
| C
|
HCHO(aq) + 2 H+ + 2 e−
|
⇌
|
CH3OH(aq)
|
0.13
|
2
|
|
| S
|
S(s) + 2 H+ + 2 e−
|
⇌
|
H
2S(g)
|
0.14
|
2
|
|
| Sn
|
Sn4+
+ 2 e−
|
⇌
|
Sn2+
|
0.15
|
2
|
|
| Cu
|
Cu2+ + e−
|
⇌
|
Cu+
|
0.159
|
1
|
|
| S
|
HSO−
4 + 3 H+ + 2 e−
|
⇌
|
SO
2(aq) + 2H2O
|
0.16
|
2
|
|
| U
|
UO2+2 + e−
|
⇌
|
UO+
2
|
0.163
|
1
|
|
| S
|
SO2−
4 + 4 H+ + 2 e−
|
⇌
|
SO
2(aq) + 2H2O
|
0.17
|
2
|
|
| Ti
|
TiO2+
+ 2 H+ + e−
|
⇌
|
Ti3+
+ H2O
|
0.19
|
1
|
|
| Sb
|
SbO+
+ 2 H+ + 3 e−
|
⇌
|
Sb(s) + H2O
|
0.2
|
3
|
|
| Fe
|
3Fe2O3(s) + 2 H+ + 2 e−
|
⇌
|
2Fe3O4(s) + H2O
|
0.22
|
2
|
|
| Ag
|
AgCl(s) + e−
|
⇌
|
Ag(s) + Cl−
|
0.22233
|
1
|
|
| As
|
H3AsO3(aq) + 3 H+ + 3 e−
|
⇌
|
As(s) + 3H2O
|
0.24
|
3
|
|
| Ru
|
Ru3+
(aq) + e−
|
⇌
|
Ru2+
(aq)
|
0.249
|
1
|
|
| Ge
|
GeO(s) + 2 H+ + 2 e−
|
⇌
|
Ge(s) + H2O
|
0.26
|
2
|
|
| U
|
UO+
2 + 4 H+ + e−
|
⇌
|
U4+
+ 2H2O
|
0.273
|
1
|
|
| Re
|
Re3+ + 3 e−
|
⇌
|
Re(s)
|
0.3
|
3
|
|
| At
|
At + e−
|
⇌
|
At-
|
0.3
|
1
|
|
| Bi
|
Bi3+
+ 3 e−
|
⇌
|
Bi(s)
|
0.308
|
3
|
|
| Cu
|
Cu2+
+ 2 e−
|
⇌
|
Cu(s)
|
0.337
|
2
|
|
| V
|
[VO]2+
+ 2 H+ + e−
|
⇌
|
V3+
+ H2O
|
0.34
|
1
|
|
| Fe
|
[Fe(CN)
6]3−
+ e−
|
⇌
|
[Fe(CN)
6]4−
|
0.3704
|
1
|
|
| Fe
|
Fc+ + e−
|
⇌
|
Fc(s)
|
0.4
|
1
|
|
| O
|
O
2(g) + 2H2O + 4 e−
|
⇌
|
4 OH−(aq)
|
0.401
|
4
|
|
| Mo
|
H
2MoO
4 + 6 H+ + 3 e−
|
⇌
|
Mo3+
+ 4H2O
|
0.43
|
3
|
|
| Ru
|
Ru2+
(aq) + 2 e−
|
⇌
|
Ru
|
0.455
|
2
|
|
| C
|
CH3OH(aq) + 2 H+ + 2 e−
|
⇌
|
CH4(g) + H2O
|
0.5
|
2
|
|
| S
|
SO
2(aq) + 4 H+ + 4 e−
|
⇌
|
S(s) + 2H2O
|
0.5
|
4
|
|
| Cu
|
Cu+
+ e−
|
⇌
|
Cu(s)
|
0.52
|
1
|
|
| C
|
CO(g) + 2 H+ + 2 e−
|
⇌
|
C(s) + H2O
|
0.52
|
2
|
|
| I
|
I−3 + 2 e−
|
⇌
|
3I−
|
0.53
|
2
|
|
| I
|
I2(s) + 2 e−
|
⇌
|
2I−
|
0.54
|
2
|
|
| Au
|
[AuI
4]−
+ 3 e−
|
⇌
|
Au(s) + 4I−
|
0.56
|
3
|
|
| As
|
H3AsO4(aq) + 2 H+ + 2 e−
|
⇌
|
H
3AsO
3(aq) + H2O
|
0.56
|
2
|
|
| Au
|
[AuI
2]−
+ e−
|
⇌
|
Au(s) + 2I−
|
0.58
|
1
|
|
| Mn
|
MnO−
4 + 2H2O + 3 e−
|
⇌
|
MnO
2(s) + 4 OH−
|
0.595
|
3
|
|
| S
|
S
2O2−
3 + 6 H+ + 4 e−
|
⇌
|
2S(s) + 3H2O
|
0.6
|
4
|
|
| Mo
|
H2MoO4(aq) + 2 H+ + 2 e−
|
⇌
|
MoO
2(s) + 2H2O
|
0.65
|
2
|
|
| C
|
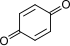 + 2 H+ + 2 e− + 2 H+ + 2 e−
|
⇌
|
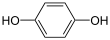
|
0.6992
|
2
|
|
| O
|
O
2(g) + 2 H+ + 2 e−
|
⇌
|
H2O2(aq)
|
0.7
|
2
|
|
| Tl
|
Tl3+
+ 3 e−
|
⇌
|
Tl(s)
|
0.72
|
3
|
|
| Pt
|
PtCl2−
6 + 2 e−
|
⇌
|
PtCl2−
4 + 2Cl−
|
0.726
|
2
|
|
| Fe
|
Fe2O3(s) + 6 H+ + 2 e−
|
⇌
|
2Fe2+ + 3H2O
|
0.728
|
2
|
|
| Se
|
H2SeO3(aq) + 4 H+ + 4 e−
|
⇌
|
Se(s) + 3H2O
|
0.74
|
4
|
|
| Pt
|
PtCl2−
4 + 2 e−
|
⇌
|
Pt(s) + 4Cl−
|
0.758
|
2
|
|
| Fe
|
Fe3+
+ e−
|
⇌
|
Fe2+
|
0.77
|
1
|
|
| Ag
|
Ag+ + e−
|
⇌
|
Ag(s)
|
0.7996
|
1
|
|
| Hg
|
Hg2+2 + 2 e−
|
⇌
|
2Hg(l)
|
0.8
|
2
|
|
| N
|
NO−3(aq) + 2 H+ + e−
|
⇌
|
NO2(g) + H2O
|
0.8
|
1
|
|
| Fe
|
2FeO2−4 + 5H2O + 6 e−
|
⇌
|
Fe
2O
3(s) + 10 OH−
|
0.81
|
6
|
|
| Au
|
[AuBr
4]−
+ 3 e−
|
⇌
|
Au(s) + 4Br−
|
0.85
|
3
|
|
| Hg
|
Hg2+
+ 2 e−
|
⇌
|
Hg(l)
|
0.85
|
2
|
|
| Ir
|
[IrCl
6]2−
+ e−
|
⇌
|
[IrCl
6]3−
|
0.87
|
1
|
|
| Mn
|
MnO−
4 + H+ + e−
|
⇌
|
HMnO−
4
|
0.9
|
1
|
|
| Hg
|
2Hg2+
+ 2 e−
|
⇌
|
Hg2+
2
|
0.91
|
2
|
|
| Pd
|
Pd2+ + 2 e−
|
⇌
|
Pd(s)
|
0.915
|
2
|
|
| Au
|
[AuCl
4]−
+ 3 e−
|
⇌
|
Au(s) + 4Cl−
|
0.93
|
3
|
|
| Mn
|
MnO
2(s) + 4 H+ + e−
|
⇌
|
Mn3+
+ 2H2O
|
0.95
|
1
|
|
| N
|
NO−3(aq) + 4 H+ + 3 e−
|
⇌
|
NO(g) + 2H2O(l)
|
0.958
|
3
|
|
| Au
|
[AuBr
2]−
+ e−
|
⇌
|
Au(s) + 2Br−
|
0.96
|
1
|
|
| Fe
|
Fe3O4(s) + 8 H+ + 2 e−
|
⇌
|
3Fe2+ + 4H2O
|
0.98
|
2
|
|
| Xe
|
[HXeO
6]3−
+ 2H2O + 2 e−
|
⇌
|
[HXeO
4]−
+ 4 OH−
|
0.99
|
2
|
|
| At
|
HAtO + H+ + e−
|
⇌
|
At + H2O
|
1.0
|
1
|
|
| V
|
[VO
2]+
(aq) + 2 H+ + e−
|
⇌
|
[VO]2+(aq) + H2O
|
1
|
1
|
|
| Te
|
H6TeO6(aq) + 2 H+ + 2 e−
|
⇌
|
TeO
2(s) + 4H2O
|
1.02
|
2
|
|
| Br
|
Br2(l) + 2 e−
|
⇌
|
2Br−
|
1.066
|
2
|
|
| Br
|
Br2(aq) + 2 e−
|
⇌
|
2Br−
|
1.0873
|
2
|
|
| Ru
|
RuO
2 + 4 H+ + 2 e−
|
⇌
|
Ru2+
(aq) + 2H2O
|
1.120
|
2
|
|
| Cu
|
Cu2+
+ 2CN−
+ e−
|
⇌
|
Cu(CN)−
2
|
1.12
|
1
|
|
| I
|
IO−
3 + 5 H+ + 4 e−
|
⇌
|
HIO(aq) + 2H2O
|
1.13
|
4
|
|
| Au
|
[AuCl
2]−
+ e−
|
⇌
|
Au(s) + 2Cl−
|
1.15
|
1
|
|
| Se
|
HSeO−
4 + 3 H+ + 2 e−
|
⇌
|
H
2SeO
3(aq) + H2O
|
1.15
|
2
|
|
| Ag
|
Ag2O(s) + 2 H+ + 2 e−
|
⇌
|
2Ag(s) + H2O
|
1.17
|
2
|
|
| Cl
|
ClO−
3 + 2 H+ + e−
|
⇌
|
ClO2(g) + H2O
|
1.18
|
1
|
|
| Xe
|
[HXeO
6]3−
+ 5H2O + 8 e−
|
⇌
|
Xe(g) + 11 OH−
|
1.18
|
8
|
|
| Pt
|
Pt2+ + 2 e−
|
⇌
|
Pt(s)
|
1.188
|
2
|
|
| Cl
|
ClO
2(g) + H+ + e−
|
⇌
|
HClO
2(aq)
|
1.19
|
1
|
|
| I
|
2IO−
3 + 12 H+ + 10 e−
|
⇌
|
I2(s) + 6H2O
|
1.2
|
10
|
|
| Cl
|
ClO−
4 + 2 H+ + 2 e−
|
⇌
|
ClO−
3 + H2O
|
1.2
|
2
|
|
| Mn
|
MnO2(s) + 4 H+ + 2 e−
|
⇌
|
Mn2+
+ 2H2O
|
1.224
|
2
|
|
| O
|
O
2(g) + 4 H+ + 4 e−
|
⇌
|
2H2O
|
1.229
|
4
|
|
| Ru
|
[Ru(bipy)
3]3+
+ e−
|
⇌
|
[Ru(bipy)3]2+
|
1.24
|
1
|
|
| Xe
|
[HXeO
4]−
+ 3H2O + 6 e−
|
⇌
|
Xe(g) + 7 OH−
|
1.24
|
6
|
|
| Tl
|
Tl3+
+ 2 e−
|
⇌
|
Tl+
|
1.25
|
2
|
|
| Cr
|
Cr
2O2−
7 + 14 H+ + 6 e−
|
⇌
|
2Cr3+
+ 7H2O
|
1.33
|
6
|
|
| Cl
|
Cl2(g) + 2 e−
|
⇌
|
2Cl−
|
1.36
|
2
|
|
| Ru
|
RuO−
4(aq) + 8 H+ + 5 e−
|
⇌
|
Ru2+
(aq) + 4H2O
|
1.368
|
5
|
|
| Ru
|
RuO4 + 4 H+ + 4 e−
|
⇌
|
RuO2 + 2H2O
|
1.387
|
4
|
|
| Co
|
CoO
2(s) + 4 H+ + e−
|
⇌
|
Co3+
+ 2H2O
|
1.42
|
1
|
|
| N
|
2NH3OH+ + H+ + 2 e−
|
⇌
|
N2H+5 + 2H2O
|
1.42
|
2
|
|
| I
|
2HIO(aq) + 2 H+ + 2 e−
|
⇌
|
I
2(s) + 2H2O
|
1.44
|
2
|
|
| Br
|
BrO−
3 + 5 H+ + 4 e−
|
⇌
|
HBrO(aq) + 2H2O
|
1.45
|
4
|
|
| Pb
|
β-PbO2(s) + 4 H+ + 2 e−
|
⇌
|
Pb2+
+ 2H2O
|
1.46
|
2
|
|
| Pb
|
α-PbO2(s) + 4 H+ + 2 e−
|
⇌
|
Pb2+
+ 2H2O
|
1.468
|
2
|
|
| Br
|
2BrO−
3 + 12 H+ + 10 e−
|
⇌
|
Br
2(l) + 6H2O
|
1.48
|
10
|
|
| Cl
|
2ClO−
3 + 12 H+ + 10 e−
|
⇌
|
Cl
2(g) + 6H2O
|
1.49
|
10
|
|
| Cl
|
HClO(aq) + H+ + 2 e−
|
⇌
|
Cl−
(aq) + H2O
|
1.49
|
2
|
|
| At
|
HAtO3 + 4 H+ + 4 e−
|
⇌
|
HAtO + 2H2O
|
1.5
|
4
|
|
| Mn
|
MnO−
4 + 8 H+ + 5 e−
|
⇌
|
Mn2+
+ 4H2O
|
1.51
|
5
|
|
| O
|
HO•
2 + H+ + e−
|
⇌
|
H2O2(aq)
|
1.51
|
1
|
|
| Au
|
Au3+
+ 3 e−
|
⇌
|
Au(s)
|
1.52
|
3
|
|
| Ru
|
RuO2−
4(aq) + 8 H+ + 4 e−
|
⇌
|
Ru2+
(aq) + 4H2O
|
1.563
|
4
|
|
| Ni
|
NiO
2(s) + 2 H+ + 2 e−
|
⇌
|
Ni2+
+ 2 OH−
|
1.59
|
2
|
|
| Ce
|
Ce4+
+ e−
|
⇌
|
Ce3+
|
1.61
|
1
|
|
| Cl
|
2HClO(aq) + 2 H+ + 2 e−
|
⇌
|
Cl
2(g) + 2H2O
|
1.63
|
2
|
|
| Ag
|
Ag
2O
3(s) + 6 H+ + 4 e−
|
⇌
|
2Ag+
+ 3H2O
|
1.67
|
4
|
|
| Cl
|
HClO2(aq) + 2 H+ + 2 e−
|
⇌
|
HClO(aq) + H2O
|
1.67
|
2
|
|
| Pb
|
Pb4+
+ 2 e−
|
⇌
|
Pb2+
|
1.69
|
2
|
|
| Mn
|
MnO−4 + 4 H+ + 3 e−
|
⇌
|
MnO2(s) + 2H2O
|
1.7
|
3
|
|
| Ag
|
AgO(s) + 2 H+ + e−
|
⇌
|
Ag+
+ H2O
|
1.77
|
1
|
|
| O
|
H2O2(aq) + 2 H+ + 2 e−
|
⇌
|
2H2O
|
1.78
|
2
|
|
| Co
|
Co3+
+ e−
|
⇌
|
Co2+
|
1.82
|
1
|
|
| Au
|
Au+
+ e−
|
⇌
|
Au(s)
|
1.83
|
1
|
|
| Br
|
BrO−
4 + 2 H+ + 2 e−
|
⇌
|
BrO−
3 + H2O
|
1.85
|
2
|
|
| Ag
|
Ag2+
+ e−
|
⇌
|
Ag+
|
1.98
|
1
|
|
| O
|
S2O2−8 + 2 e−
|
⇌
|
2SO2−4
|
2.01
|
2
|
|
| O
|
O3(g) + 2 H+ + 2 e−
|
⇌
|
O
2(g) + H2O
|
2.075
|
2
|
|
| Mn
|
HMnO−
4 + 3 H+ + 2 e−
|
⇌
|
MnO
2(s) + 2H2O
|
2.09
|
2
|
|
| Xe
|
XeO
3(aq) + 6 H+ + 6 e−
|
⇌
|
Xe(g) + 3H2O
|
2.12
|
6
|
|
| Xe
|
H
4XeO
6(aq) + 8 H+ + 8 e−
|
⇌
|
Xe(g) + 6H2O
|
2.18
|
8
|
|
| Fe
|
FeO2−4 + 8 H+ + 3 e−
|
⇌
|
Fe3+
+ 4H2O
|
2.2
|
3
|
|
| Xe
|
XeF
2(aq) + 2 H+ + 2 e−
|
⇌
|
Xe(g) + 2HF(aq)
|
2.32
|
2
|
|
| Xe
|
H
4XeO
6(aq) + 2 H+ + 2 e−
|
⇌
|
XeO
3(aq) + 3H2O
|
2.42
|
2
|
|
| F
|
F2(g) + 2 e−
|
⇌
|
2F−
|
2.87
|
2
|
|
| F
|
F2(g) + 2 H+ + 2 e−
|
⇌
|
2HF(aq)
|
3.05
|
2
|
|
| Tb
|
Tb4+ + e–
|
⇌
|
Tb3+
|
3.1
|
1
|
|
| Pr
|
Pr4+ + e–
|
⇌
|
Pr3+
|
3.2
|
1
|
|
| Kr
|
KrF2(aq) + 2 e−
|
⇌
|
Kr(g) + 2F−
(aq)
|
3.27
|
2
|
|
Más resultados...
